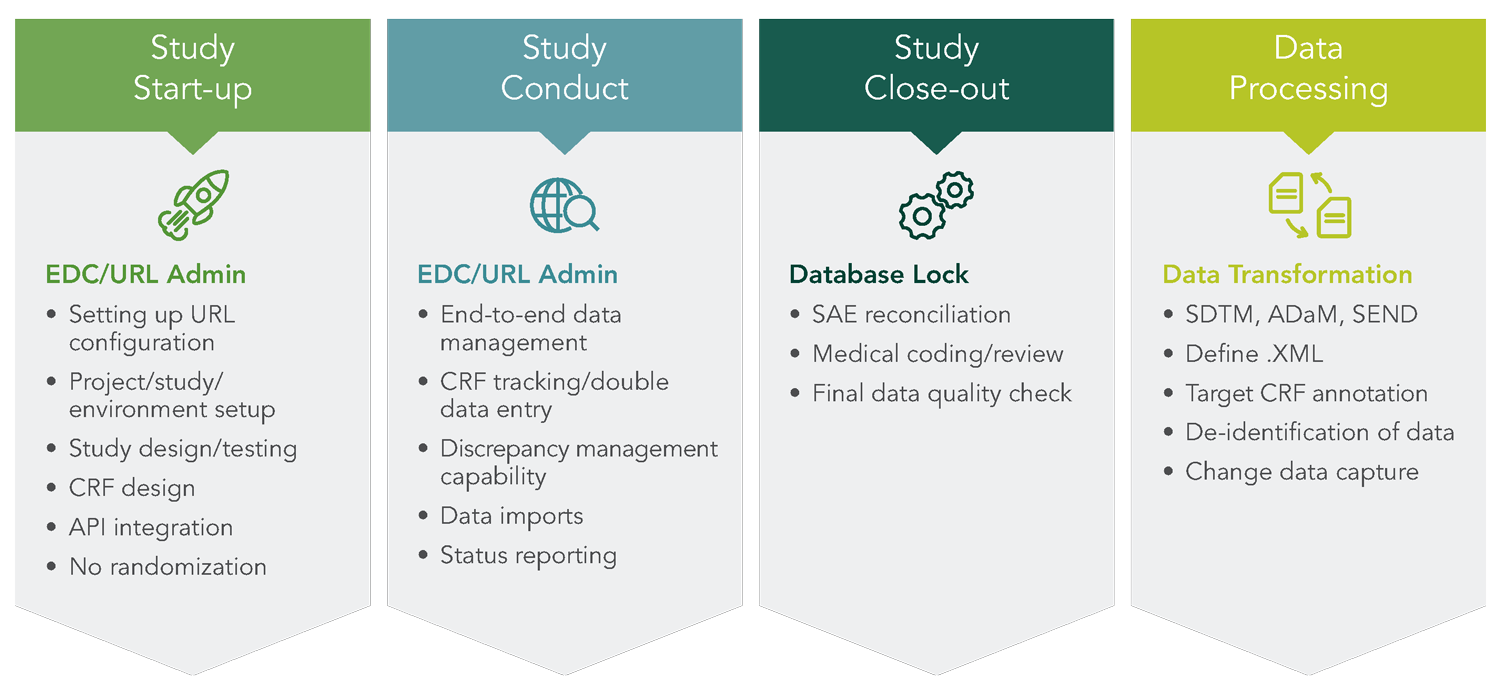
You need customized data management solutions tailored to your specific needs for any study in any therapeutic area. George Clinical’s data management team has industry experience across contract research organizations, biotech, medical device
and pharmaceutical companies and includes certified database builders and medical coders. Therapeutically, the team aligns with the company’s scientific leaders in our core areas of renal, oncology, cardiology, respiratory, as well as experience in many other areas including medical devices studies.
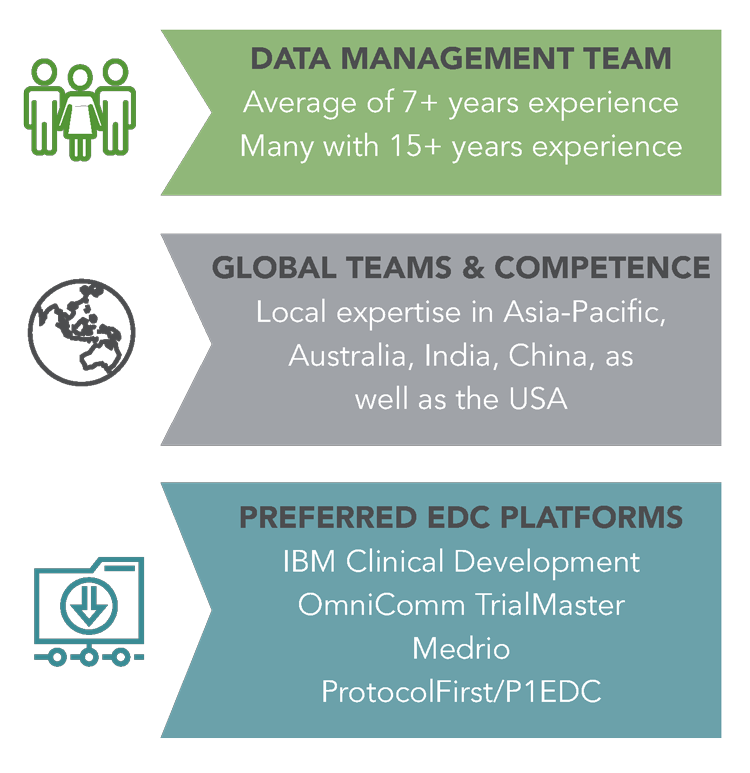
As one of the leading data management teams in the Asia-Pacific region with staff located in Australia, Europe, India and China as as well as the USA, George Clinical can truly operate across all time zones with local representation. As a contract research organization (CRO) with offices in Asia, USA and Europe that engages some of the world’s most distinguished scientific expertise in a diverse range of therapeutic areas, we are able to leverage our scientific leadership to establish and maintain unique investigator networks across the globe.
George Clinical specializes in the development and maintenance of databases for a wide range of study types, from simple questionnaires to large global registration studies, which are customized to each study’s unique requirements. We can deliver the cost-effective solutions both academic and commercial customers need for epidemiology, registration and post-registration studies. Teams spread across the Asia-Pacific region, Australia, Europe, India and China with the addition of a USA team mean that you are getting a solution specific to each region with experts in local practices and customs. Our quality-by-design approach uses a risk-based methodology and adheres to SOPs for all standard clinical data management tasks ensuring consistency and quality in data management services across studies and regions. We provide our customers with end-to-end data management services for Phase I–IV and other post-marketing commitments, as well as tailored services for academic research trials. The fully validated and 21 CFR Part 11 compliant systems George Clinical use include IBM® Clinical Development, OmniComm® TrialMaster and Medrio.