George Clinical has a culture founded on Quality Management embedded across all our activities. Our Quality Management System facilitates a harmonized approach to the delivery and maintenance of quality and compliance which includes all the aspects in the following diagram:
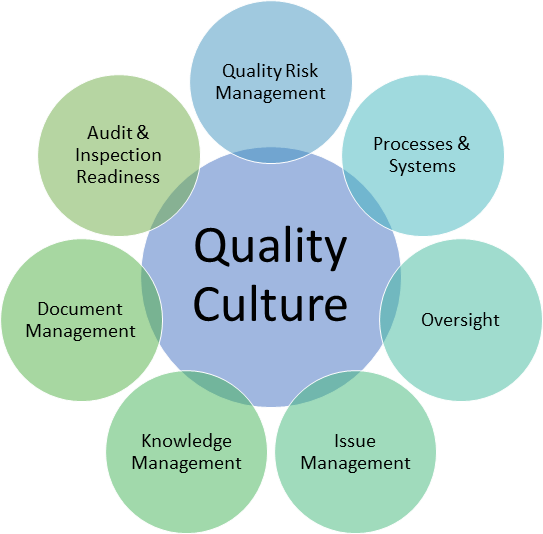
We work with you on your projects to comply with the Guideline for ICH Good Clinical Practice (GCP) and local regulatory requirements to protect participants and ensure data integrity. We implement our quality management approach through all phases of the project from first contact with the client through study closure and reporting.
George Clinical provides global coverage of Clinical Research Compliance and Training, and Quality Assurance with people in Australia, China, East Asia, India and the USA
George Clinical conducts and maintains:
George Clinical provides quality assurance from design to delivery.