
Bringing Excellence | Oncology Strengths | Navigating CGT Complexities | Leadership | Awards & Recognition
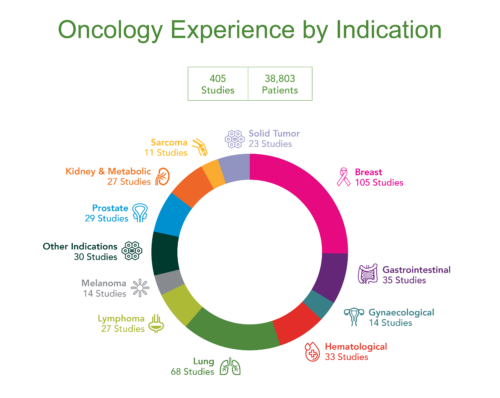
George Clinical, a leading provider of oncology trial, scientific and operational solutions throughout Asia Pacific, USA and Europe, has conducted more than 400 clinical trials in solid and hematological tumors, having recruited more than 38,000 oncology trial participants at more than 2,200 sites across the globe. George Clinical has the breadth of expertise and clinical networks to make what is traditionally one of the most complex and time-consuming therapeutic areas to conduct clinical trials one of the most streamlined.
We base our clinical research model upon scientific leadership. George Clinical scientific and clinical thought leaders have the expertise to provide effective clinical trial design and can engage directly with significant investigator networks, placing projects as priorities with site teams in multiple oncology therapeutic areas. Our scientific leadership model excels in motivating and engaging investigators to boost recruitment and retention in especially challenging patient populations.
Bringing Excellence to Oncology Studies
With any new trial, George Clinical provides an analysis of any foreseeable barriers, issues, risks identified regarding subject enrollment, or study timelines and accordingly provides mitigation activities, transforming challenges into opportunities.
- Site selection – Our experience has taught us how important selecting the right sites will be to deliver the required patients in the defined timelines. George Clinical has vast oncology experience and can draw from this experience to ascertain the best sites for your trial. Our teams work closely with sponsors to ensure the careful vetting of all sites.
- Operational risk management – Our teams assess tight start-up and recruitment timelines for potential sites based on historical performance and work closely with each site to develop a comprehensive recruitment plan prior to site activation.
- Patient Retention – Our oncology project teams ensure a complete and comprehensive follow-up of study participants from the very beginning of the trial to ensure the collection of complete outcome data.
George Clinical oncology strengths include:
- Proven leadership: An extensive list of global scientific leaders
- Real-world experience: Deep operational experience with oncology studies
- The best geographies: A regional advantage with an extensive site network founded in the Asia-Pacific region, the largest incremental recruitment region for oncology studies in the world
- Strong patient network: A vast database and network of potential patients for immediate screening and recruitment
- Flexibility: The ability to expand to additional study sites as needed
- High quality data: Our milestone-driven teams remain focused on delivering top-quality data sets
In addition to the study specific service offerings, we also provide the following advantages to our clients and partners:
- Cost savings – George Clinical budgets are designed with “economy of scale” to ensure a maximum cost savings. Our project teams understand the need to deliver a cost-effective, reliable project on time and on budget for clients.
- Tailored, service delivery, experience, and staff quality – Our organization is well positioned to manage studies empowered by a strong history of scientific leadership. All assigned team members will be George Clinical employees with the appropriate experience, local knowledge of the selected sites and standard practices to ensure the long-term success of these programs.
- Timely, accurate and complete communication – Project leads understand the time-sensitive nature of each study and have a sterling reputation of “on-time” service delivery and client satisfaction. Communication is paramount and George Clinical team members ensure effective communication to anticipate challenges and provide seamless delivery, all while minimizing cost.
Navigating Cell and Gene Therapy Trial Complexity
George Clinical brings a CGT team with more than 13 years experience.
Trial Experience
- Phase I autologous tumor infiltrating lymphocytes in melanoma
- Phase I allogeneic CAR-T in CLL
- Phase IIb/III autologous cell gene therapies using apheresis centers
- Phase I/II T-cell autologous therapy
- Phase I irradiated autologous tumor cells loaded with medical device
- Whole-process management for CAR-T therapy
Success Drivers in CGT Trials
- Established KOL network and site relationships
- Access to specialized labs for testing
- Robust scientific and regulatory expertise
- IP handling and management
- Globalized QMS and flexible service options
CGT Study Management Services
- Project management and study execution
- Site identification and monitoring
- Regulatory consultancy and strategy
- Portfolio and protocol design
- KOL network and regional scientific services
- Quality management system
- Vendor selection and oversight
- Risk-based project management
- Data management science
- Safety management
- Medical science and medical writing
- Quality assurance
Meet Our Oncology Scientific Leadership:
Working through all phases of clinical research, our team of leading scientific leaders and operational experts connect key opinion leaders, investigators, research sites, patients, regulators and clients with a common purpose and an innovative operational platform for conducting oncology clinical trials.
Read About Our Industry Awards & Recognition:
George Clinical has been recognized by industry experts including Frost & Sullivan, Citeline and Informa Pharma Intelligence.

